Preimplantation Genetic Diagnosis (PGD/PGS) Technologies
After biopsy, the removed cells must be analyzed using one of the available PGD methods. A list detailing the currently used PGD technologies clearly shows that no technology is perfect, each one has its specific drawbacks and limitations. For instance, while we believe that OGS technology is more reliable when it comes to aneuploidy testing, Microarray-based PGD methods have a potential for providing an unlimited amount of genetic information from a single cell.
| FISH | 24 Chromosome Capable | EGS | |||
| aCGH | SNP | OGS | |||
| First Clinical Use | 1991 | 2003 | 2008 | 2009 | 2011 |
| 24 Chromosome Aneuploidy Screening | no | yes | yes | yes | no |
| Detects Haploid, Triploid, & Polyploid Embryos | yes | no | yes/no | yes | yes |
| Detection of Abnormal Karyokinesis | yes | no | no | yes | yes |
| Detection of Mitotic Trisomies / Mosaicism | yes | yes | no | yes | yes |
| Detection of Uniparental Disomies | no | no | yes | no | no |
| Ease of Sample Preparation | difficult | easy | easy | dificult | easiest |
| Sample Preparation Procedure | fixation | tubing | tubing | fixation | sliding |
| Procedure Duration | 3-12 hrs | 12 hrs | 12 hrs | 6 hrs | 1 hr |
FISH
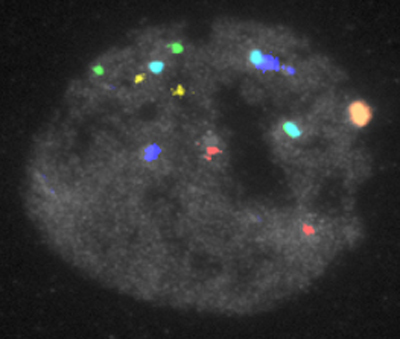
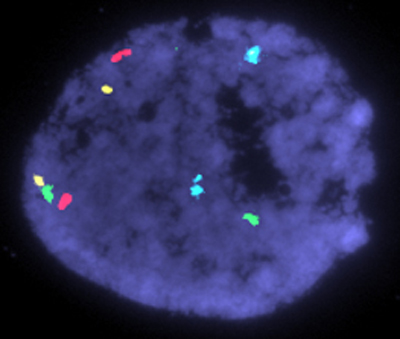
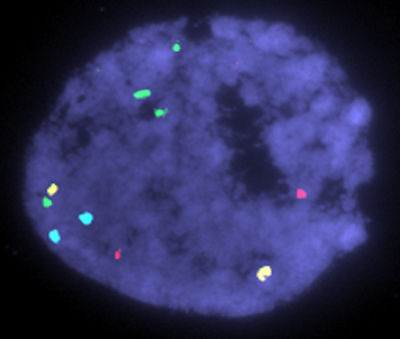
During Fluorescent In Situ Hybridization (FISH) the biopsied blastomere is fixed so that the cytoplasm is washed away leaving only the nucleus attached to the slide. Then the probes are hybridized to the nucleus. The probes are the strands of DNA identical to some specific regions of human chromosomes. For example, the probe for chromosome 18 has the same polynucleotide sequence as a centromeric DNA of chromosome 18. The probes are labeled with fluorophores and the results of FISH are detected by a fluorescent microscope. Standard FISH is capable of detecting up to 12 chromosomes in a few hours.
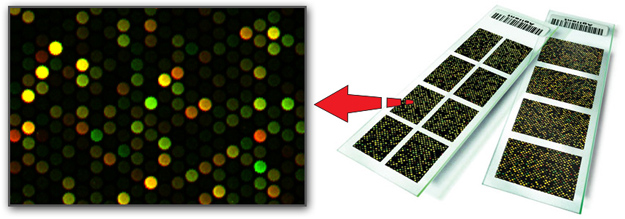
DNA Microarray Technology
Also known as DNA Chips, this is basically FISH technology turned upside-down. Instead of hybridizing labeled DNA probes to the nucleus fixed on the slide, the probes are pre-attached to the slide (thus the name, array of DNA probes) and DNA from the biopsied cell nucleus is restricted into pieces, labeled by fluorophores and then hybridized to the microarray. The concept of Fluorescent In-Situ Hybridization (FISH) stays the same, but now we are producing fluorophore-labeled probes out of the cell nucleus to hybridize these "embryonic" probes to the microarray. After hybridization the microarray is scanned by a laser and analyzed by a computer. Microarrays are capable of analyzing all 24 human chromosomes in less than 24 hours.
As the name implies, microarray is small, but an individual cell nucleus is still smaller! Thus the First Challenge: the need to amplify DNA from a single nucleus using polymerase chain reaction (PCR) specifically tailored for the whole genome amplification (WGA). There are few ways for the WGA, but none of them is perfect: some parts of the whole genome are always overamplified and others are underrepresented. This leads to pseudo-duplications or pseudo-deletions following microarray analysis. The only reliable way to avoid this problem is to have more than one cell for PGD analysis. For this reason, microarray-based PGD is especially recommended for patients who choose blastocyst biopsy.
The Second Challenge is to make sense out of the hybridized microarray, because just hybridizing "embryonic" probes to an array is not very informative. If the analyzed cell is totally missing chromosomes 21 (so-called Nullisomy 21) microarray will definitely reveal this by showing no signals at the spots specific for chromosome 21. However, probes made from a cell with trisomy or monosomy for chromosome 21 will hybridize to microarray as readily as probes made from a normal cell: all array spots will be covered and be fluorescing during scanning.
There are two conceptually different ways of getting aneuploidy revealed by microarrays: using tested and proven CGH (Comparative Genome Hybridization) or the new and promising SNP (Single Nucleotide Polymorphism) technologies. As a result, there are two types of microarrays, aCGH and SNP.
Array CGH
aCGH stands for array Comparative Genome Hybridization. Following WGA, sample DNA is labeled with Red fluorophore and applied to aCGH microarray along with normal male DNA labeled with Green fluorophore. Both tested DNA in Red and control DNA in Green compete with each other for DNA targets on the microarray. By analyzing the color of each spot, it is possible to determine if the analyzed nucleus has the same amount of DNA as the normal control (thus also being normal) or outperforms the control DNA (thus has an extra genetic material, like trisomy 21). If the tested nucleus does not have a single chromosome 21 then the respective spots on the microarray will fluoresce with Green only.
aCGH made its debut in the PGD field with BAC (bacterial artificial chromosomes) based arrays, designed specifically for PGD use. Quite recently the leaders in microarray technology took notice of PGD and entered the field by either buying the original PGD-oriented microarray technology or by offering highly competitive products of their own. One of these new developments is the so-called High Density Multiplex arrays.
At ViaGene we are using custom 8x60k SurePrint G3 Human CGH arrays from Agilent Technologies. The numbers mean that a single microscope slide (the base for all microarrays on the market) has 8 individual arrays printed on it, with each array consisting of 60,000 "spots". One individual spot has millions of copies of long oligonucleotide printed on it, oligonucleotide specific for some unique region of human chromosome. The arrays are optimized for PGD aneuploidy screening, they were developed by ViaGene and produced by Agilent Technologies specifically for PGD aneuploidy testing.
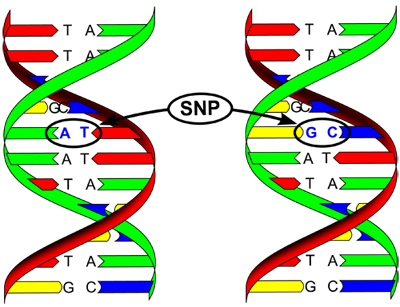
SNP Microarray
SNP stands for Single Nucleotide Polymorphism. The concept of SNP is as different from aCGH as aCGH is different from FISH. SNP is a variation of single nucleotides in human population and is the most frequent type of variation in the genome. SNP Microarrays are made of very short DNA probes, called short oligonucleotides, these are the only probes capable of distinguishing the variants of SNPs and thus making a call regarding the genetics of a cell. The technology is very new, very powerful and potentially the most promising. It is not yet known how SNP Microarray compares to the more established aCGH technologies used for PGD. A controversy is also raging regarding the need of "parental support technology" to make SNP microarrays applicable to PGD.
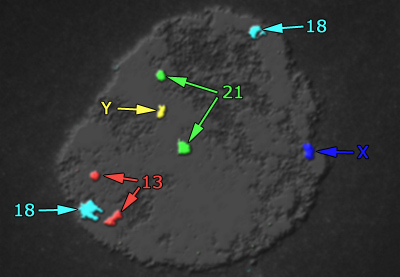
OGS
In Oligonucleotide Genome Screening (OGS), as in FISH, the nucleus is used as a target for the fluorophore-labeled probes. However, the similarity to FISH ends here. The probes themselves are completely different: OGS uses oligonucleotide probes developed originally for microarray use. The protocol for target pretreatment, hybridization and wash was totally reinvented in order to completely eliminate the unavoidable drawbacks of FISH, namely "failed hybridizations" and "target degradation". Unlike FISH and Microarray, which require 3-12 and 6-16 hours of hybridization, hybridization time for OGS is just 5 minutes per cycle.
Because OGS does not depend on PCR/WGA, it is not affected by DNA contamination and thus provides more reliable results. Unlike Microarray-based PGD, OGS easily detects triploids and polyploids, genetic defects responsible for almost 10% of genetically abnormal first-trimester abortions. Although less time-consuming than Microarray, the procedure is much more labor-intensive and requires significantly more training. With OGS technology aneuploidy screening of all 24 chromosomes takes less than 6 hours.
EGS
Express Genome Screening (EGS) was specifically designed to be faster and less complicated than any other PGD technology on the market today. EGS relies on the techniques developed for OGS for the chromosomal analysis, but there are significant differences in the sample preparation procedure and the speed of analysis. Where OGS requires a difficult to master fixation procedure, which limits its availability throughout the country, EGS uses a new 'sliding' procedure which can be easily taught to any embryologist within minutes. This allows clinics who were previously limited to offering only Microarray based PGD to now also offer EGS.
However, the speed of the EGS procedure is what really sets it apart. EGS can provide results 12 times faster than any of the currently available Microarray techniques. In most cases EGS results are available in about an hour, while Microarray based methods require 12 or more hours.
ViaGene offers EGS-XY, specifically designed for gender selection cases, and EGS-5, designed to screen for ALL types of congenital defects related to chromosomal euploidy, including Down's Syndrome, Patau's Syndrome, Edwards Syndrome, Trisomy X, Turner Syndrome, XYY Syndrome, and Klinefelter Syndrome.
Future of PGD
Microarray-based PGD is not "The Ultimate PGD," it is just the latest idea in the PGD field, and the technology itself is still in its Research and Development (R&D) phase. Moreover, it is not one, but actually a few technologies with the "Microarray" name tag. At this time we are witnessing a fierce competition between different types of Microarrays, only time will tell whether it is aCGH, or SNP-based microarrays, BAC or high-density oligos, or parental support technology or karyomapping that will become the next PGD standard. There are indications that Genome Sequencing may be the Next Best Thing. Also, lets not forget that the same companies making microarrays are also developing new generations of better probes for the "golden standard" in PGD: the tried and true FISH. This means that our own OGS and EGS methods are constantly improving!